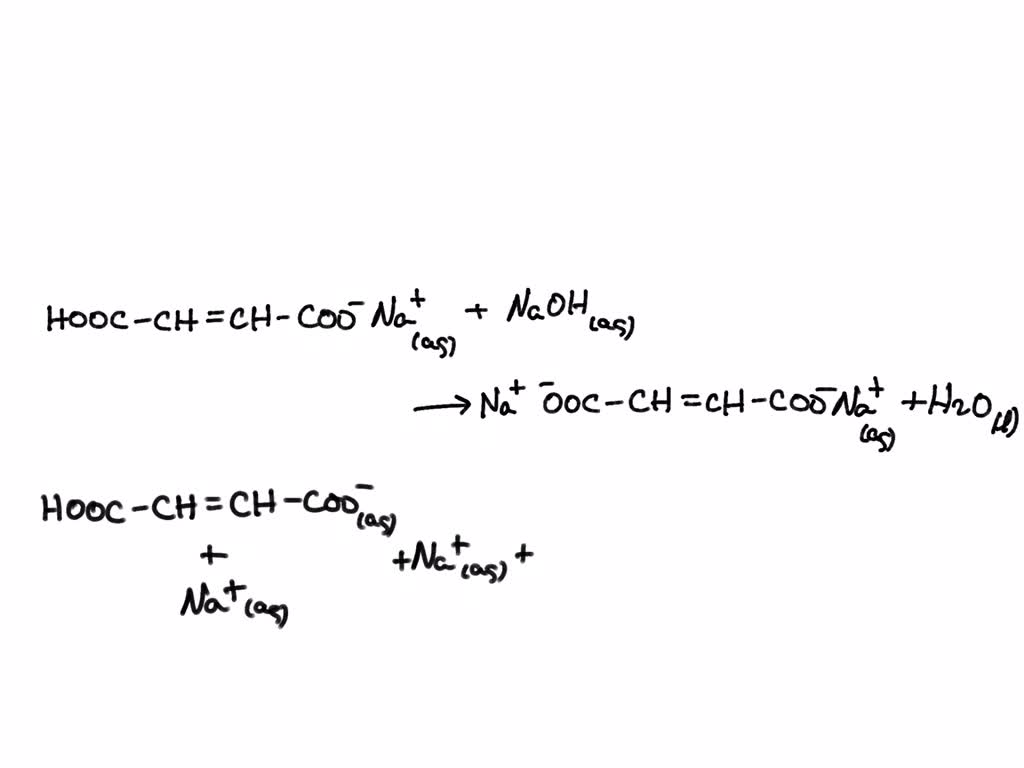
Titration Balanced Net Ionic Equation . the balanced molecular equation is: Write the equation in terms of all of the ions in the solution. there are three steps to writing a net ionic equation: In other words, break all of the strong electrolytes into the ions they form in aqueous solution. Apply the nernst equation in all directions to determine. a net ionic equation is the most accurate representation of the actual chemical process that occurs. Balancing by mass means making sure that there are equal numbers of. Enter an equation of an ionic chemical equation and press the balance button. calculate net ionic equation. net ionic equations must be balanced by both mass and charge. write balanced oxidation/reduction equations. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical reactions occurring in aqueous.
from www.numerade.com
calculate net ionic equation. Enter an equation of an ionic chemical equation and press the balance button. net ionic equations must be balanced by both mass and charge. In other words, break all of the strong electrolytes into the ions they form in aqueous solution. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical reactions occurring in aqueous. a net ionic equation is the most accurate representation of the actual chemical process that occurs. the balanced molecular equation is: Balancing by mass means making sure that there are equal numbers of. Write the equation in terms of all of the ions in the solution. there are three steps to writing a net ionic equation:
SOLVED Write the balanced and net ionic equation for the reaction
Titration Balanced Net Ionic Equation In other words, break all of the strong electrolytes into the ions they form in aqueous solution. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical reactions occurring in aqueous. Balancing by mass means making sure that there are equal numbers of. the balanced molecular equation is: Apply the nernst equation in all directions to determine. calculate net ionic equation. Enter an equation of an ionic chemical equation and press the balance button. Write the equation in terms of all of the ions in the solution. a net ionic equation is the most accurate representation of the actual chemical process that occurs. write balanced oxidation/reduction equations. there are three steps to writing a net ionic equation: In other words, break all of the strong electrolytes into the ions they form in aqueous solution. net ionic equations must be balanced by both mass and charge.
From www.chegg.com
Solved 187. Write balanced net ionic equations for the Titration Balanced Net Ionic Equation Write the equation in terms of all of the ions in the solution. write balanced oxidation/reduction equations. Balancing by mass means making sure that there are equal numbers of. Enter an equation of an ionic chemical equation and press the balance button. there are three steps to writing a net ionic equation: the balanced molecular equation is:. Titration Balanced Net Ionic Equation.
From www.numerade.com
SOLVED Redox Titrations of Hydrogen Peroxide Write balanced net ionic Titration Balanced Net Ionic Equation a net ionic equation is the most accurate representation of the actual chemical process that occurs. Balancing by mass means making sure that there are equal numbers of. write balanced oxidation/reduction equations. there are three steps to writing a net ionic equation: Write the equation in terms of all of the ions in the solution. net. Titration Balanced Net Ionic Equation.
From celjgvoh.blob.core.windows.net
Balanced Equation For The Titration at Barbara Hulbert blog Titration Balanced Net Ionic Equation Write the equation in terms of all of the ions in the solution. write balanced oxidation/reduction equations. Enter an equation of an ionic chemical equation and press the balance button. the balanced molecular equation is: a net ionic equation is the most accurate representation of the actual chemical process that occurs. Hcl(aq) + naoh(aq) → nacl(aq) +. Titration Balanced Net Ionic Equation.
From www.numerade.com
SOLVED Write a balanced complete molecular equation and net ionic Titration Balanced Net Ionic Equation Apply the nernst equation in all directions to determine. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical reactions occurring in aqueous. a net ionic equation is the most accurate representation of the actual chemical process that occurs. the balanced molecular equation is: Enter an equation of an ionic chemical equation and press the balance button. Write the. Titration Balanced Net Ionic Equation.
From slideplayer.com
Net Ionic Equations Balance the equation ppt download Titration Balanced Net Ionic Equation net ionic equations must be balanced by both mass and charge. Balancing by mass means making sure that there are equal numbers of. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical reactions occurring in aqueous. the balanced molecular equation is: write balanced oxidation/reduction equations. Enter an equation of an ionic chemical equation and press the balance. Titration Balanced Net Ionic Equation.
From studylib.net
Net Ionic Equations Titration Balanced Net Ionic Equation a net ionic equation is the most accurate representation of the actual chemical process that occurs. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical reactions occurring in aqueous. the balanced molecular equation is: net ionic equations must be balanced by both mass and charge. Apply the nernst equation in all directions to determine. Enter an equation. Titration Balanced Net Ionic Equation.
From www.youtube.com
Precipitation Reactions & Net Ionic Equations Chemistry YouTube Titration Balanced Net Ionic Equation Write the equation in terms of all of the ions in the solution. the balanced molecular equation is: Apply the nernst equation in all directions to determine. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical reactions occurring in aqueous. In other words, break all of the strong electrolytes into the ions they form in aqueous solution. a. Titration Balanced Net Ionic Equation.
From www.numerade.com
SOLVED Write the balanced and net ionic equation for the reaction Titration Balanced Net Ionic Equation Apply the nernst equation in all directions to determine. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical reactions occurring in aqueous. Balancing by mass means making sure that there are equal numbers of. Enter an equation of an ionic chemical equation and press the balance button. In other words, break all of the strong electrolytes into the ions they. Titration Balanced Net Ionic Equation.
From study.com
Representing Changes in Matter with a Balanced Chemical or Net Ionic Titration Balanced Net Ionic Equation Balancing by mass means making sure that there are equal numbers of. there are three steps to writing a net ionic equation: In other words, break all of the strong electrolytes into the ions they form in aqueous solution. the balanced molecular equation is: write balanced oxidation/reduction equations. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical. Titration Balanced Net Ionic Equation.
From www.youtube.com
Net Ionic Equations AcidBase, PPT, Gas Forming YouTube Titration Balanced Net Ionic Equation calculate net ionic equation. there are three steps to writing a net ionic equation: write balanced oxidation/reduction equations. In other words, break all of the strong electrolytes into the ions they form in aqueous solution. net ionic equations must be balanced by both mass and charge. Write the equation in terms of all of the ions. Titration Balanced Net Ionic Equation.
From www.youtube.com
net ionic equations strong bases and weak acids YouTube Titration Balanced Net Ionic Equation there are three steps to writing a net ionic equation: Write the equation in terms of all of the ions in the solution. net ionic equations must be balanced by both mass and charge. Enter an equation of an ionic chemical equation and press the balance button. Hcl(aq) + naoh(aq) → nacl(aq) + h 2o(l) chemical reactions occurring. Titration Balanced Net Ionic Equation.
From www.chegg.com
Solved Write a balanced net ionic equation for the reaction Titration Balanced Net Ionic Equation a net ionic equation is the most accurate representation of the actual chemical process that occurs. Enter an equation of an ionic chemical equation and press the balance button. calculate net ionic equation. write balanced oxidation/reduction equations. there are three steps to writing a net ionic equation: Write the equation in terms of all of the. Titration Balanced Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for H2O2 + KMnO4 + H2SO4 YouTube Titration Balanced Net Ionic Equation write balanced oxidation/reduction equations. net ionic equations must be balanced by both mass and charge. calculate net ionic equation. Apply the nernst equation in all directions to determine. a net ionic equation is the most accurate representation of the actual chemical process that occurs. the balanced molecular equation is: there are three steps to. Titration Balanced Net Ionic Equation.
From printableleperenardmu.z21.web.core.windows.net
Describe A Neutralization Reaction Titration Balanced Net Ionic Equation Write the equation in terms of all of the ions in the solution. calculate net ionic equation. net ionic equations must be balanced by both mass and charge. the balanced molecular equation is: there are three steps to writing a net ionic equation: write balanced oxidation/reduction equations. Apply the nernst equation in all directions to. Titration Balanced Net Ionic Equation.
From fr.slideserve.com
PPT Common Types of Reactions PowerPoint Presentation, free download Titration Balanced Net Ionic Equation Write the equation in terms of all of the ions in the solution. there are three steps to writing a net ionic equation: the balanced molecular equation is: a net ionic equation is the most accurate representation of the actual chemical process that occurs. net ionic equations must be balanced by both mass and charge. Balancing. Titration Balanced Net Ionic Equation.
From www.numerade.com
SOLVED What is the balanced net ionic equations of the acidbase Titration Balanced Net Ionic Equation net ionic equations must be balanced by both mass and charge. a net ionic equation is the most accurate representation of the actual chemical process that occurs. Apply the nernst equation in all directions to determine. Balancing by mass means making sure that there are equal numbers of. there are three steps to writing a net ionic. Titration Balanced Net Ionic Equation.
From www.slideserve.com
PPT Writing Net Ionic Equations PowerPoint Presentation, free Titration Balanced Net Ionic Equation there are three steps to writing a net ionic equation: net ionic equations must be balanced by both mass and charge. Balancing by mass means making sure that there are equal numbers of. In other words, break all of the strong electrolytes into the ions they form in aqueous solution. calculate net ionic equation. the balanced. Titration Balanced Net Ionic Equation.
From www.numerade.com
Calculate the value of the equilibrium constant for the balanced net Titration Balanced Net Ionic Equation a net ionic equation is the most accurate representation of the actual chemical process that occurs. Balancing by mass means making sure that there are equal numbers of. there are three steps to writing a net ionic equation: net ionic equations must be balanced by both mass and charge. Write the equation in terms of all of. Titration Balanced Net Ionic Equation.